OMNI® SURGICAL SYSTEM
with one intelligent device


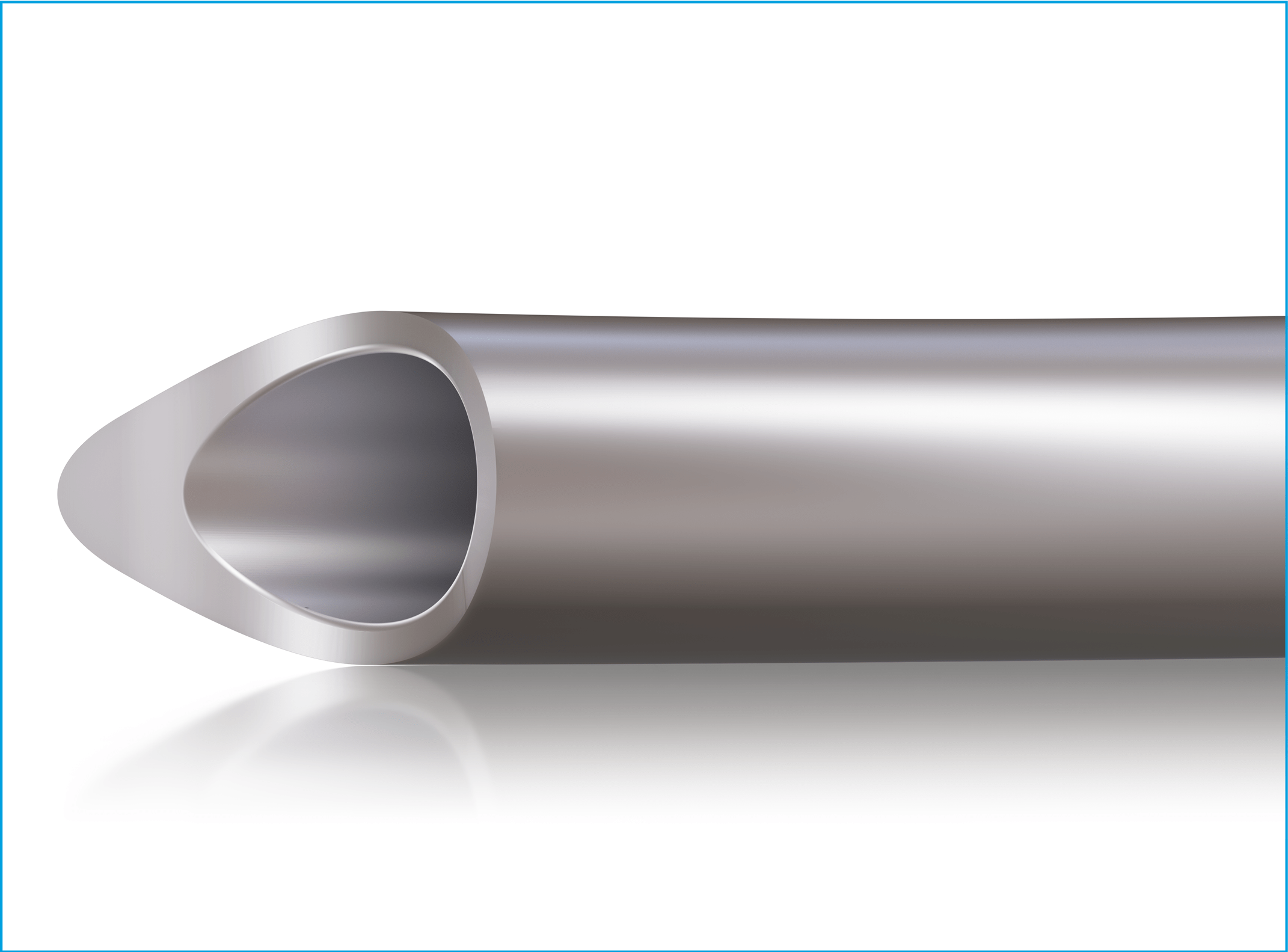
The cannula tip has a new profile that enables gentle and precise access to Schlemm’s canal while continuing to enable full 360-degree catheterization associated with the OMNI procedure.
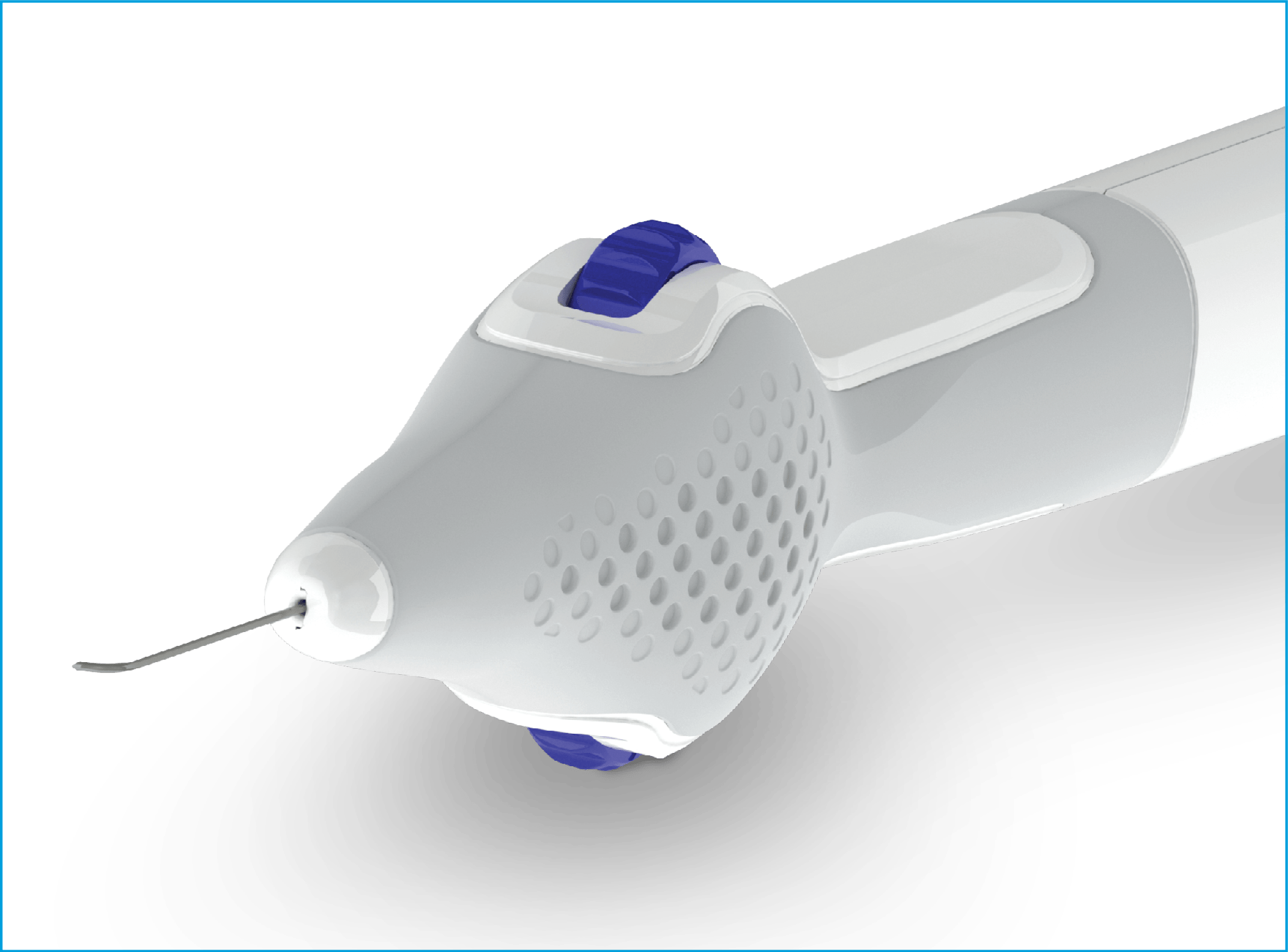
New enhancements enable surgeons to more easily rotate and position the cannula tip within Schlemm’s canal with precise finger rotations versus wrist adjustments.
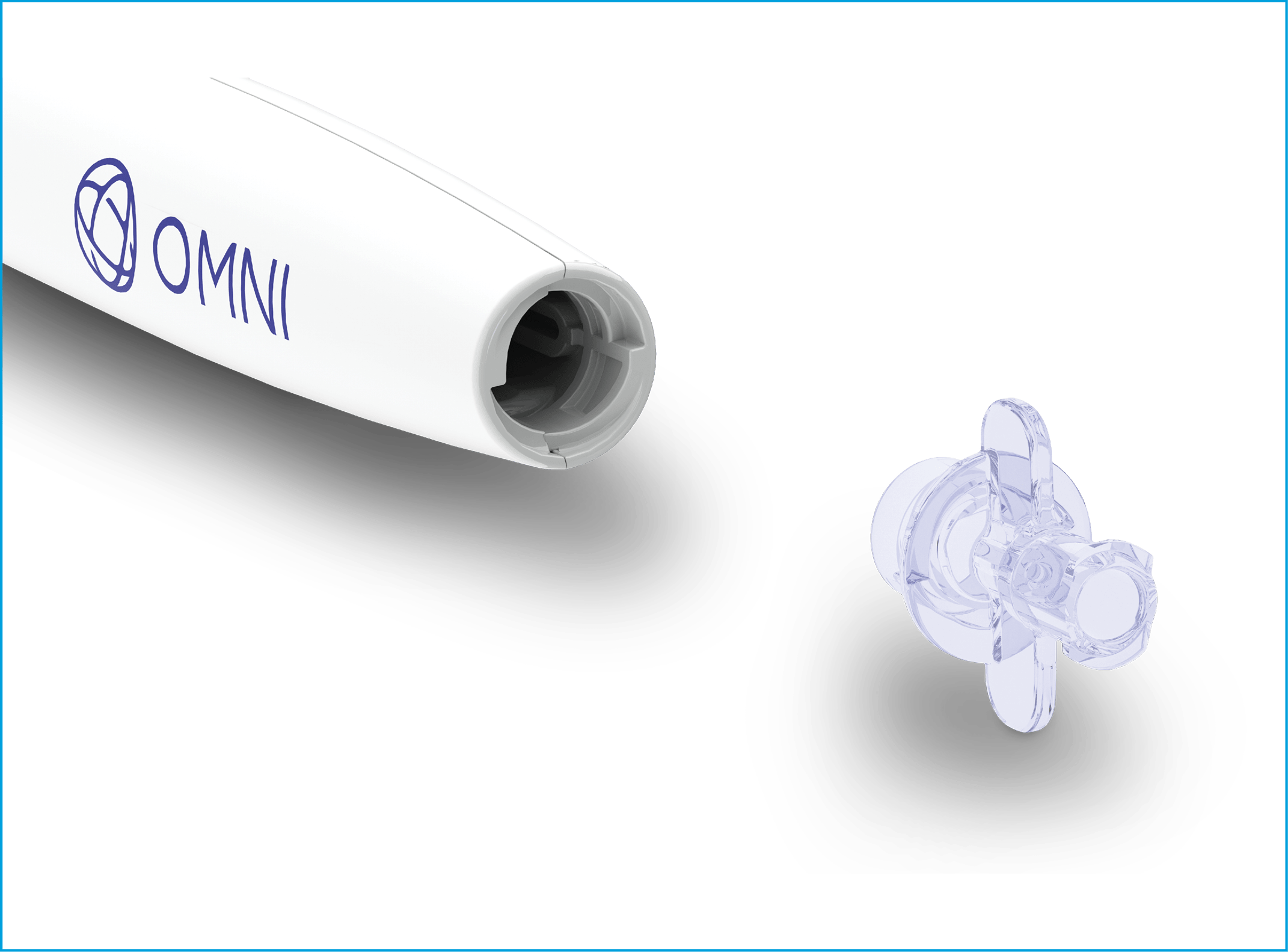
The handle’s viscoelastic Luer connector is now removable and detaches when the viscoelastic cartridge is removed, resulting in more clearance with the surgical microscope.
The OMNI® Surgical System is indicated for the catheterization and transluminal viscodilation of Schlemm’s canal and the cutting of trabecular meshwork to reduce intraocular pressure in adult patients with open-angle glaucoma.
1. Do not use the OMNI in any situations where the angle is compromised or has been damaged (e.g., from trauma or surgery), since it may not be possible to visualize the angle or to properly pass the microcatheter.
2. Do not use the OMNI in patients with angle recession; neovascular glaucoma; chronic angle closure; narrow-angle glaucoma; traumatic or malignant glaucoma; or narrow inlet canals with plateau iris.
1. Do not use in cases where there is insufficient visibility to properly see the angle. The following conditions may prohibit sufficient visualization of the angle required for safe and successful cannula placement: corneal edema, corneal haze, corneal opacity, or any other conditions that may inhibit gonioscopic view of the angle and intended cannula entry location.
2. Perform gonioscopy prior to taking a patient to surgery to exclude congenital anomalies of the angle, anterior segment dysgeneses, peripheral anterior synechiae (PAS), rubeosis, and any other angle abnormalities that could lead to improper placement of the cannula and microcatheter and pose a hazard.
3. Maintain direct microscopic or gonioscopic visualization of the cannula tip and microcatheter tip at all times during the procedure to facilitate advancement and to avoid inadvertently damaging intraocular structures, kinking the microcatheter or unintended tearing of trabecular meshwork.
Adverse events that may be reasonably associated with the use of the OMNI® System in the eye include but are not limited to the following: anterior chamber shallowing, severe, prolonged, or persistent intraocular inflammation including toxic anterior segment syndrome (TASS), endophthalmitis or other ocular infection, aqueous misdirection, Descemet’s membrane tear or detachment, intracorneal hematoma, choroidal effusion, suprachoroidal hemorrhage, corneal decompensation, corneal injury, corneal edema or opacification, unintended trabeculotomy, cyclodialysis cleft, hyphema, hypopyon, hypotony, hypotony maculopathy, IOL dislocation, cataract formation, iris injury, tear, or iridodialysis, loss of vitreous, perforation of sclera, posterior capsular bag rupture, proliferative vitreoretinopathy, pupillary block, pupillary membrane formation, retinal detachment, retinal dialysis, retinal flap tears, secondary surgical intervention, including but not limited to glaucoma surgery, and vitreous hemorrhage.
We appreciate your interest in the OMNI Surgical System. A member of our team will review your enquiry and get back to you as soon as possible during business hours.